|
|
|
..................Transgenic Animals...............
A transgenic mouse (or any other species) is simply an organism that has had DNA introduced into one or more of its cells artificially. This is commonly done in one of two ways. DNA can be integrated in a random fashion by injecting it into the pronucleus of a fertilized ovum. In this case, the DNA can integrate anywhere in the genome, and multiple copies often integrate in a head-to-tail fashion. There is no need for homology between the injected DNA and the host genome.
Targeted insertion, the other common method of producing transgenic animals, is accomplished by introducing the DNA into embryonic stem (ES) cells and selecting for cells in which the DNA has undergone homologous recombination with matching genomic sequences. For this to occur, there must be several kilobases of homology between the exogenous and genomic DNA, and positive selectable markers (e.g., antibiotic resistance genes) must be included. In addition, negative selectable markers (e.g., "toxic" genes) are often used to select against cells that have incorporated DNA by non-homologous recombination (i.e., random insertion).
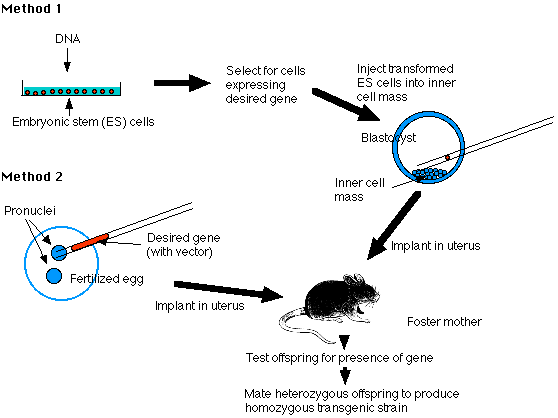
Pronuclear injection of DNA is often used to characterize the ability of a promoter to direct tissue-specific gene expression. For example, promoter/enhancer constructs may be used to drive expression of a reporter gene, such as LacZ, whose protein product, beta-galactosidase, is detected histochemically. Another major use for transgenic mice produced by pronuclear injection of DNA is to examine the effects of overexpressing and misexpressing endogenous or foreign genes at specific times and locations in the animal.
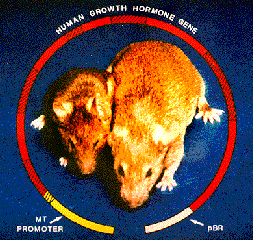
Many factors influence whether a promoter/transgene construct will express (produce the appropriate mRNA and protein) in transgenic mice. The promoters that are used must be known to function appropriately in vivo (in vitro function does not always guarantee this). Transgene constructs may have accumulated mutations during cloning (especially if PCR was involved). Perhaps the most important consideration has to do with the trangene's insertion site in the mouse genome. At many chromosomal locations, transgenes will be transcriptionally silent. At others they may express, but with a tissue- and temporal specificity that is not identical to what has previously been seen with the same promoter construct. The intrinsic ability of a promoter construct to drive transgene expression reliably and with faithful tissue specificity also varies from promoter to promoter, for reasons that are not well understood. For these reasons, the TMF can guarantee to produce mice that have integrated the injected DNA, but we cannot make guarantees about transgene expression
One of the most common uses of targeted insertion of DNA is to make "knockout mice". Typically, homologous recombination is used to insert a selectable gene (e.g., neo, which confers G418 (neomycin) resistance) driven by a constitutive promoter (e.g., PGK) into an essential exon of the gene one wishes to disrupt (often the first coding exon). To accomplish this, the neo gene (or other DNA) is flanked by large stretches of DNA (on the order of 2-7 kb) that exactly match the genomic sequences surrounding the desired insertion point. Once this construct is electroporated into ES cells, the cells' own machinery performs the homologous recombination. To make it possible to select against ES cells that incorporate DNA by non-homologous recombination, it is common for targeting constructs to include a negatively selectable gene outside the region intended to undergo recombination (typically the gene is cloned adjacent to the shorter of the two regions of genomic homology). Because DNA lying outside the regions of genomic homology is lost during homologous recombination, cells undergoing homologous recombination cannot be selected against, whereas cells undergoing random integration of DNA often can. A commonly used gene for negative selection is the herpes virus thymidine kinase gene, which confers sensitivity to the drug gancyclovir
Once positive ES clones have been grown up and frozen, the production of transgenic animals can begin. Donor females are mated, blastocysts are harvested, and 10-15 ES cells are injected into each blastocyst. Eight to ten blastocysts are then implanted into a uterine horn of each pseudopregnant recipient. By choosing the appropriate donor strain, the detection of chimeric offspring (i.e., those in which some fraction of tissue is derived from the transgenic ES cells) can be as simple as observing hair and/or eye color. If the transgenic ES cells do not contribute to the germline (sperm or eggs), the transgene cannot be passed on to offspring. The TMF cannot guarantee that ES cells will "go germline", but, if handled properly (e.g. not allowed to become differentiated or aneuploid in vitro), those ES lines that are in common use generally contribute to the germline an acceptable percentage of the time.
It is important to note (and perhaps not obvious) that production of transgenic mice by pronuclear injection can also occasionally result in a mosaic animal. This happens when integration of the transgene is delayed until after the first cell division. There can also be multiple insertion events at different genomic loci and at different times. Thus, a single founder can be mosaic for one insertion site but not the other.
Results:
If the replacement gene (A* in the diagram) is nonfunctional (a "null" allele), mating of the heterozygous transgenic mice will produce a strain of "knockout mice" homozygous for the nonfunctional gene (both copies of the gene at that locus have been knocked out").
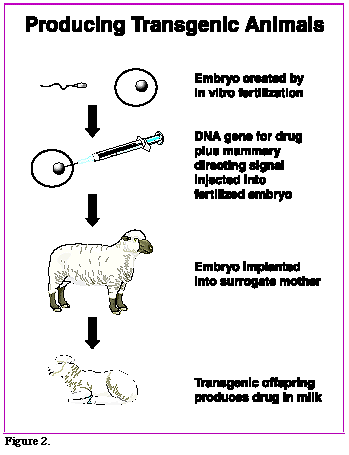
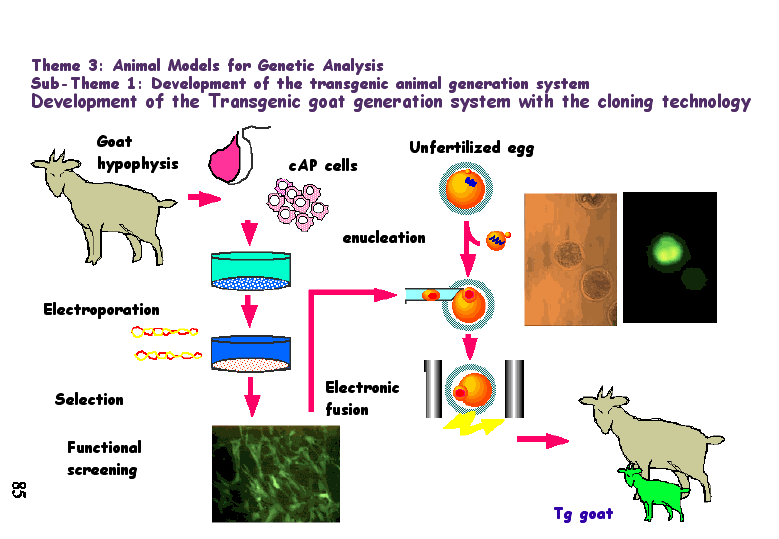
Knockout mice are valuable tools for discovering the function(s) of genes for which mutant strains were not previously available. Two generalizations have emerged from examining knockout mice:Until recently, the transgenes introduced into sheep inserted randomly in the genome and often worked poorly. However, in July 2000, success at inserting a transgene into a specific gene locus was reported. The gene was the human gene for alpha1-antitrypsin, and two of the animals expressed large quantities of the human protein in their milk.
This is how it was done.
Sheep fibroblasts growing in tissue culture were treated with a vector that contained these segments of DNA:
This locus was chosen because fibroblasts secrete large amounts of collagen and thus one would expect the gene to be easily accessible in the chromatin.
Some people inherit two non- or poorly-functioning genes for this protein. Its resulting low level or absence produces the disease Alpha1-Antitrypsin Deficiency (A1AD or Alpha1). The main symptoms are damage to the lungs (and sometimes to the liver).
Preliminary results from both methods indicate that it may be possible for chickens to produce as much as 0.1 g of human protein in each egg that they lay.
Not only should this cost less than producing therapeutic proteins in culture vessels, but chickens will probably add the correct sugars to glycosylated proteins - something that E. coli cannot do.
Transgenic pigs have also been produced by fertilizing normal eggs with sperm cells that have incorporated foreign DNA. This procedure, called sperm-mediated gene transfer (SMGT) may someday be able to produce transgenic pigs that can serve as a source of transplanted organs for humans
notice :the images were selected from many sites.